You've Been Briefed
When was Zantac recalled? In April of 2020, the FDA announced that all Zantac brand and its generic equivalent heartburn drugs, both prescription and over-the-counter versions, should be immediately pulled from the market. Why was Zantac pulled off the Market? On September 13, 2019, the U.S. Food and Drug Administration (FDA) reported the discovery of the carcinogenic contaminant N-nitrosodimethylamine (NDMA) in Zantac (ranitidine). NDMA was found in Zantac and its generic equivalents at levels beyond what is considered safe. Scientific research has shown that, when ranitidine comes in contact with water, it creates a chemical reaction that causes the formation of NDMA. Studies have linked the NDMA to cancer in animals and humans. What is NMDA? A carcinogenic contaminant N-nitrosodimethylamine (NDMA) is a carcinogenic contaminant that has been linked to cancer in patients that have taken the drug. NDMA is a volatile, combustible, yellow, oily liquid nitrosamine with a faint characteristic odor. The chemical decomposes when exposed to light and emits toxic fumes when heated. NDMA is used primarily in research labs induce tumors in experimental animals. In 2014, the EPA issued a statement about NDMA contamination in drinking water. What are the lawsuits about? In the current litigation, the plaintiffs allege that the manufacturers placed on the market a dangerous product and failed to warn consumers of the serious risks and side effects. The main manufacturers of Zantac include: Sanofi, Boehringer Ingelheim, and GSK. What cancers and other diseases are linked to Zantac (Ranitidine)? Bladder Colon Pancreatic Esophageal Kidney Liver Stomach/Gastric Breast Lung Prostate What do I do if me of someone I know took Zantac and now has cancer? Contact WolfEdlund, P.C. Immediately
Because many of the early signs of mesothelioma are the same as those caused by other problems, most people with mesothelioma have symptoms for at least a few months before they are diagnosed. The American Cancer Society indicates the following symptoms are associated with Mesothelioma. Symptoms associated with respiratory and lungs are Pleural mesothelioma (mesothelioma of the chest): Pain in the side of the chest or lower back Shortness of breath Cough Trouble swallowing (feeling like food gets stuck) Hoarseness Swelling of the face and arms Symptoms associated with the abdomen, stomach and digestion are referred to as Peritoneal mesothelioma symptoms. Abdominal (belly) pain Swelling or fluid in the abdomen Nausea and vomiting Constipation Symptoms associated with the heart are referred to as Pericardial mesothelioma symptoms: Chest pain Irregular heart rhythm Heart murmur Shortness of breath General mesothelioma symptoms can also include: Fever Excessive sweating Fatigue Weight loss (without trying) Blood clots Loss of appetite These symptoms can be caused by mesothelioma, but more often they are caused by other conditions. However, if you believe that you have been exposed to asbestos or have a loved one that was exposed to asbestos, we suggest you contact your health care provider right away.

Mesothelioma is a malignant tumor that is caused by inhaled asbestos fibers and forms in the lining of the lungs, abdomen or heart. Symptoms can include shortness of breath and chest pain. The life expectancy for most mesothelioma patients is approximately 12 months after diagnosis. Asbestos is the only known cause of Mesothelioma. Who Is at Risk for Asbestos Exposure? People who work in the following occupations: Miners Factory Workers. Insulation Manufacturers and Installers Railroad workers Automotive workers Shipyard workers Gas Mask manufacturers Plumbers Boilermakers Mechanics HVAC workers Pipe fitters Construction workers, including sheet rock and flooring installation crews Power plant workers Electricians The American Cancer Society states that, “Family members of people exposed to asbestos at work can also be exposed because the workers can carry home asbestos fibers on their clothes.” You may be eligible to seek compensation for mesothelioma from working with asbestos. If you would like to speak with a mesothelioma lawyer, please contact us immediately.
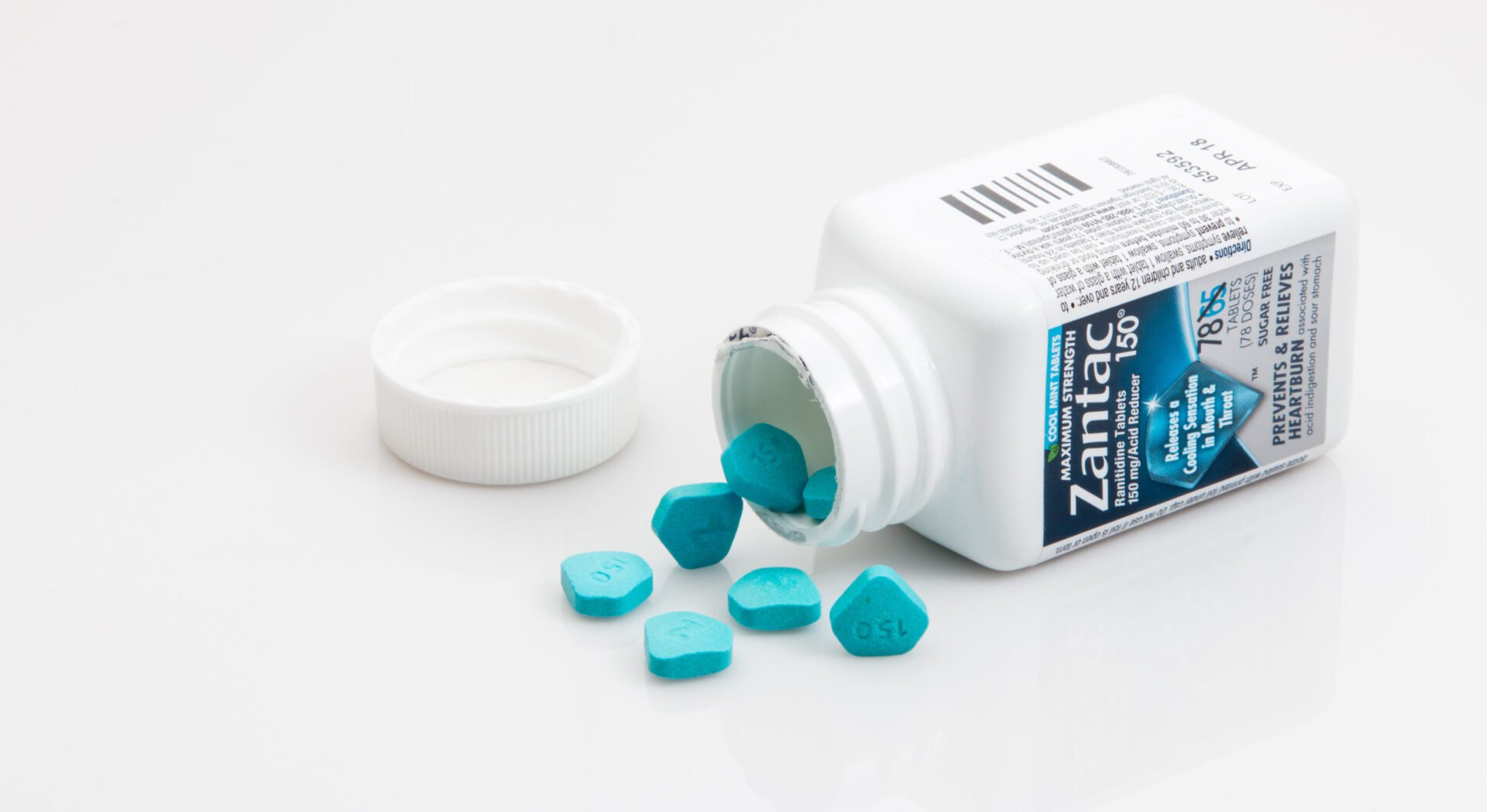
Do all Zantac products contain Ranitidine? All Zantac branded products are linked to cancer. These are the most common Zantac products containing ranitidine: Zantac 150 Tablets Zantac 150 Maximum Strength Zantac 150 Maximum Strength Cool Mint Zantac 75 Tablets These products are all linked to Birth defects and the following cancers: Bile Duct Cancer Brain Cancer Bladder cancer Breast Cancer Colorectal (Colon) cancer Esophageal cancer Kidney cancer Liver cancer Lung cancer Non-Hodgkins Lymphoma Ovarian cancer Pancreatic cancer Prostate cancer Small Intestine cancer Stomach cancer Thyroid cancer Uterine cancer If you or a loved one have taken Zantac and have been diagnosed with one or more of the cancers listed above, call our Antacid Trial Attorneys for a lawsuit consultation.
Ranitidine is a drug that has been manufactured, sold “over the counter” and prescribed by doctors, for heartburn acid reflux, and other digestive disorders to millions of Americans in 1983. The most common brand name for Ranitidine is Zantac. Zantac was produced by many drug companies such as Pfizer, GlaxoSmithKline, Sanofi, Boehringer, add more here with links Zantac was first sold in the United States in 1983. By 1987, Zantac had become one of the most widely used drug on the market and was the first drug in the U.S. to top $1B in sales. In June 2019, a pharmaceutical testing lab found that Zantac and other generic versions of Ranitidine contained dangerously high levels of N-nitrosodimethylamine (NDMA). NDMA is highly toxic to humans, especially the liver, and has been conclusively linked to various forms of cancer since the 1970s. Dimethylhydrazine, which is the "D" in NDMA, is a component of rocket fuel. Recent testing reported that a single tablet of Zantac contained 26,000 times the acceptable daily limit of NDMA according to the FDA. These findings were reported to the FDA in June 2019 . In fall 2019, the FDA issued a public safety warning on the medications containing Ranitidine. Voluntary recalls began and continued through the fall. The FDA did further testing and in April 2020, the FDA removed the product from the market. If you took Zantac and have been diagnosed with cancer, please complete the form to determine if you may be eligible for compensation. Upon review of the form, our Ranitidine Injury Lawyers will contact you for a free consultation.

After studying over 200 research papers on NDMA and the NDMA cancer risk, the International Agency for Cancer Research (IARC), a unit of the World Health Organization (WHO), placed the chemical in the “Group 2A” category, which indicates that a substance is “probably carcinogenic to humans.” The agency found that NDMA is “carcinogenic in all animal species tested” and noted that the metabolism of NDMA by humans and animals is similar. NDMA was found to cause malignant tumor growth in multiple organs, in multiple species, by multiple routes of exposure (oral, inhalation, subcutaneous (under the skin), injections), with dose-response relationships appearing in several studies. Scientists are also gaining detailed knowledge of the biochemical mechanisms used by the body to repair NDMA DNA damage and how those mechanisms play a role in the progression towards cancer. The IARC is comprised of teams of scientists from multiple countries who investigate the cancer-causing potential of suspect chemicals and substances and publish papers to report the results.
The most common type of surgical mesh is made from polypropylene, a flexible, woven plastic that can be cut into different sizes and shapes. In addition to common surgical risks —infection, anesthesia side effects, pain or swelling at the wound site— surgical mesh has some known complications and higher complex risks associated with it. Documented issues linked to hernia mesh surgery include: Mesh erosion or rejection Mesh shrinkage or migration Nerve damage Fistulas or tissue erosion Bowel perforation and obstruction Large or Small Intestine blockage or obstruction Fluid build-up at surgical site (seroma) Hole in neighboring tissues or organs In addition, there have been numerous adverse reactions reported to the FDA. Click here to view the FDA Site for Mesh Product Complications . If you or a loved one suffer side effects such as: pain, bleeding, fluid buildup, or bowel obstruction following a hernia mesh surgery, contact us today.

